"Executive Summary North America Drug Safety Solutions And Pharmacovigilance Market :
Data Bridge Market Research analyses that the drug safety solutions and pharmacovigilance market will exhibit a CAGR of around 13.85% for the forecast period of 2021-2028.
With North America Drug Safety Solutions And Pharmacovigilance Market research report it becomes easy to develop a successful Market strategy for the business. To formulate this excellent Market report, a combination of best industry insight, practical solutions, talent solutions and latest technology have been employed. This industry analysis report speaks in detail about the manufacturing process, type and applications. The market data analysed and evaluated in this market report makes achieve the business goals and objectives in preset time frame. An appropriate utilization of recognized statistical tools and coherent models for analysis and forecasting of market data makes North America Drug Safety Solutions And Pharmacovigilance Market report outshining.
North America Drug Safety Solutions And Pharmacovigilance Market report presents top to bottom examination of the market for estimating income, return on investment (ROI) and developing business strategies. Market shares of key players in the major areas of the globe such as Europe, North America, Asia Pacific, South America, Middle East and Africa are also studied. Here, market analysis makes an assessment of the expected rise, growth or fall of the product in the specific forecast period. An analytical assessment of the competitors confers clear idea of the most important challenges faced by them in the present market and in upcoming years.
Discover the latest trends, growth opportunities, and strategic insights in our comprehensive North America Drug Safety Solutions And Pharmacovigilance Market report. Download Full Report: https://www.databridgemarketresearch.com/reports/north-america-drug-safety-solutions-and-pharmacovigilance-market
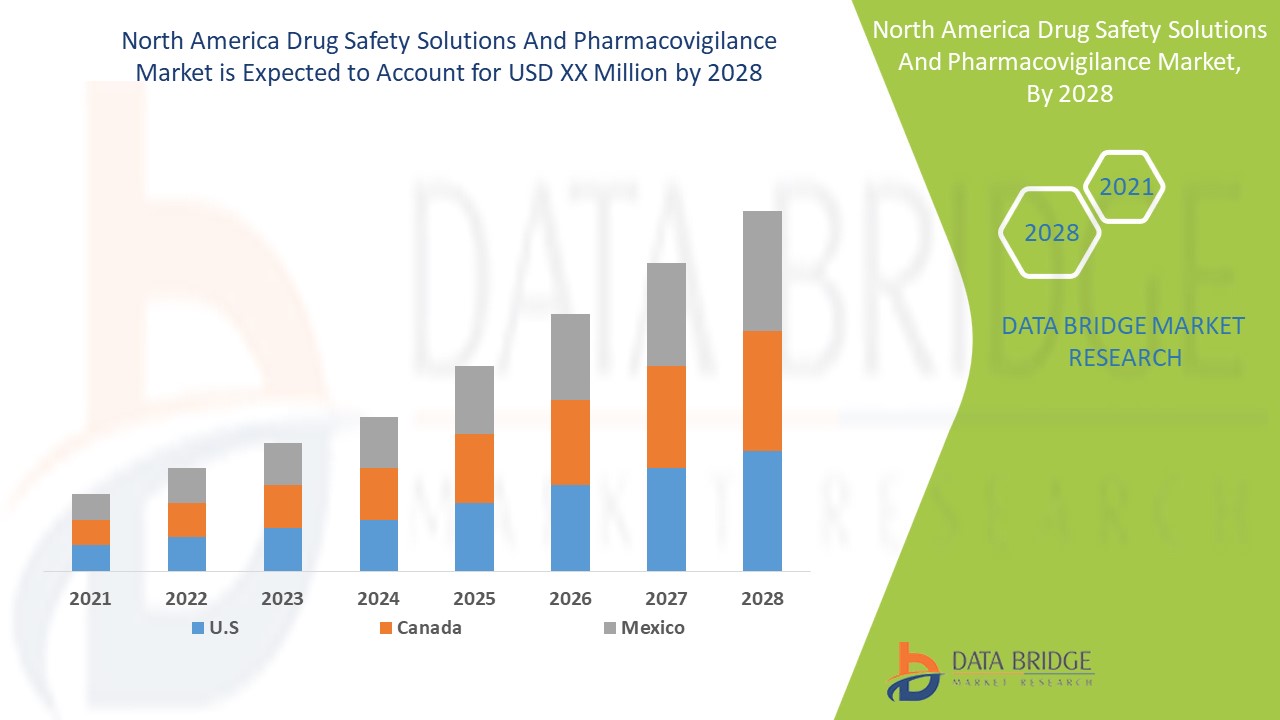
North America Drug Safety Solutions And Pharmacovigilance Market Overview
**Segments**
- The North America drug safety solutions and pharmacovigilance market can be segmented on the basis of type, service, method, end-user, and country. By type, the market can be categorized into adverse event reporting software, drug safety audits software, issue tracking software, fully integrated software, and others. In terms of services, the market can be divided into in-house and outsourced services. Based on the method, the market is classified into spontaneous reporting, intensified ADR reporting, targeted spontaneous reporting, cohort event monitoring, and EHR mining. Furthermore, the end-users of drug safety solutions and pharmacovigilance in North America include pharmaceutical and biotechnology companies, contract research organizations (CROs), business process outsourcing (BPO) firms, and pharmacovigilance consulting companies.
**Market Players**
- Some of the key market players in the North America drug safety solutions and pharmacovigilance market include Oracle Corporation, SAS Institute Inc., Ennov, Linical Accelovance, United Biosource Corporation, Capgemini, TAKE Solutions Limited, Cognizant, IQVIA, ArisGlobal, EXTEDO, Online Business Applications Inc., and Sparta Systems, among others. These companies are actively involved in product development, strategic collaborations, mergers and acquisitions, and geographic expansions to strengthen their market presence and cater to the growing demand for drug safety solutions and pharmacovigilance services in North America. With a focus on innovation, technological advancements, and regulatory compliance, these market players strive to offer comprehensive solutions that enhance drug safety and optimize pharmacovigilance practices in the region.
North America's drug safety solutions and pharmacovigilance market is witnessing significant growth due to several factors impacting the healthcare industry landscape. One key trend shaping the market is the increasing emphasis on regulatory compliance and patient safety. Regulatory bodies are imposing stringent guidelines and regulations to ensure the efficacy and safety of pharmaceutical products, driving the demand for robust drug safety solutions and pharmacovigilance services. Market players are aligning their efforts to develop innovative solutions that meet these regulatory requirements and address the evolving needs of the pharmaceutical and biotechnology companies operating in North America.
Another factor fueling the market growth is the rising adoption of advanced technologies such as artificial intelligence (AI), machine learning, and big data analytics in pharmacovigilance practices. These technologies enable efficient data analysis, early detection of adverse events, and predictive risk assessment, enhancing the overall drug safety measures and improving patient outcomes. Market players are investing significantly in R&D activities to incorporate these technologies into their solutions, offering cutting-edge pharmacovigilance services to their clients in North America.
Moreover, the increasing prevalence of chronic diseases and the growing complexity of drug development processes are driving the demand for comprehensive drug safety solutions in the region. With the rising number of clinical trials and drug approvals, there is a heightened focus on monitoring and managing adverse events throughout the product lifecycle. Drug safety solutions and pharmacovigilance services play a crucial role in detecting, assessing, and mitigating potential risks associated with drug therapies, contributing to the overall success of pharmaceutical companies in North America.
Furthermore, the market is witnessing a shift towards outsourced pharmacovigilance services, as pharmaceutical companies seek specialized expertise and cost-effective solutions to manage their pharmacovigilance activities. Outsourcing allows companies to leverage the capabilities of external service providers, streamline operations, and focus on core competencies, driving the adoption of outsourced drug safety solutions in North America. Market players are expanding their service portfolios to meet this growing demand for outsourced pharmacovigilance services, offering customized solutions that align with the specific requirements of their clients.
Overall, the North America drug safety solutions and pharmacovigilance market presents lucrative opportunities for market players to capitalize on the evolving healthcare landscape and address the increasing complexities associated with drug safety and regulatory compliance. By leveraging technology, industry expertise, and strategic partnerships, companies can enhance their market presence, drive innovation, and deliver value-added solutions that contribute to the advancement of drug safety practices in the region.The North America drug safety solutions and pharmacovigilance market is poised for significant growth driven by various factors that are shaping the healthcare landscape in the region. One of the key drivers propelling the market growth is the increasing focus on regulatory compliance and patient safety. Regulatory bodies are tightening guidelines to ensure the safety and efficacy of pharmaceutical products, prompting companies to adopt robust drug safety solutions and pharmacovigilance services to comply with these regulations and meet patient safety standards. This emphasis on compliance is creating a demand for innovative solutions that can address the evolving needs of pharmaceutical and biotechnology companies in North America.
Additionally, the market is witnessing a surge in the adoption of advanced technologies such as artificial intelligence, machine learning, and big data analytics in pharmacovigilance practices. These technologies are revolutionizing the way adverse events are detected, analyzed, and managed, leading to more efficient drug safety processes and improved patient outcomes. Market players are investing heavily in research and development to integrate these technologies into their offerings, providing cutting-edge pharmacovigilance services to clients in the region.
Furthermore, the increasing prevalence of chronic diseases and the complexity of drug development processes are contributing to the growing demand for comprehensive drug safety solutions in North America. As the number of clinical trials and drug approvals rises, there is a heightened need for effective monitoring and management of adverse events throughout the product lifecycle. Drug safety solutions and pharmacovigilance services are vital for detecting, assessing, and mitigating potential risks associated with drug therapies, thereby supporting the success of pharmaceutical companies in the region.
Moreover, the market is experiencing a shift towards outsourced pharmacovigilance services as companies look for specialized expertise and cost-effective solutions to manage their pharmacovigilance activities. By outsourcing these services, pharmaceutical firms can optimize operations, tap into external capabilities, and concentrate on core competencies, which is driving the adoption of outsourced drug safety solutions in North America. Market players are diversifying their service offerings to cater to this increasing demand for outsourced pharmacovigilance services, providing tailored solutions that meet the specific needs of their clients.
In conclusion, the North America drug safety solutions and pharmacovigilance market presents lucrative opportunities for companies to navigate the evolving healthcare landscape, address the complexities related to drug safety and regulatory compliance, and enhance their market presence through innovation, advanced technology integration, and strategic collaborations. By staying abreast of industry trends, leveraging expertise, and offering value-added solutions, market players can drive growth, deliver enhanced drug safety practices, and contribute to the advancement of pharmacovigilance standards in North America.
The North America Drug Safety Solutions And Pharmacovigilance Market is highly fragmented, featuring intense competition among both global and regional players striving for market share. To explore how global trends are shaping the future of the top 10 companies in the keyword market.
Learn More Now: https://www.databridgemarketresearch.com/reports/north-america-drug-safety-solutions-and-pharmacovigilance-market/companies
DBMR Nucleus: Powering Insights, Strategy & Growth
DBMR Nucleus is a dynamic, AI-powered business intelligence platform designed to revolutionize the way organizations access and interpret market data. Developed by Data Bridge Market Research, Nucleus integrates cutting-edge analytics with intuitive dashboards to deliver real-time insights across industries. From tracking market trends and competitive landscapes to uncovering growth opportunities, the platform enables strategic decision-making backed by data-driven evidence. Whether you're a startup or an enterprise, DBMR Nucleus equips you with the tools to stay ahead of the curve and fuel long-term success.
This comprehensive report provides:
- Improve strategic decision making
- Research, presentation and business plan support
- Show emerging North America Drug Safety Solutions And Pharmacovigilance Marketopportunities to focus on
- Industry knowledge improvement
- It provides the latest information on important market developments.
- Develop an informed growth strategy.
- Build technical insight
- Description of trends to exploit
- Strengthen competitor analysis
- By providing a risk analysis, you can avoid pitfalls that other companies may create.
- Ultimately, you can maximize your company's profitability.
Browse More Reports:
North America Healthcare Advertising Market
Global Smart Antimicrobial Healthcare Coatings and Surfaces Market
Europe Blow-Fill-Seal Equipment Market
North America Flat glass Market
Global Traditional Toys and Games Market
Global Acrylic Monomers Market
Global Pallet Box Market
Global Flooring Chemical Market
Global Smart Greenhouse Market
Middle East and Africa Agricultural Lubricants Market
Global Hermetic Packaging Market
Global Pressure Sensitive Self-Adhesive Labeler Equipment Market
Europe Acute Coronary Syndrome Market
North America Nuclear Imaging Devices Market
Global Butterfly Needle Sets Market
Global Personal Finance Management Market
Asia-Pacific Mycotoxin Testing Market
Global Pie Mixes Market
Global Battery Thermal System Market
Global Lipase Market
Middle East and Africa Flexible Sensors Market
Global Brake Sensors Market
Global Cloud Gaming Market
Global Gaming Consoles Market
Global Competent Cells Market
North America Left Ventricular Assist Device (LVAD) Market
Global Drawplate Market
Global Customer Experience Platforms Market
Europe Sulfuric Acid Market
Canada Private Label Food and Beverages Market
Global Pre-press for Packaging Market
Global Medical Foods for Inborn Errors of Metabolism Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com
Tag
"


